<em>Sarcocystis sp.</em> parasites in the Mexican Great-tailed Grackle (<em>Quiscalus mexicanus</em>), Bronzed Cowbird (<em>Molothrus aeneus</em>), and Stripe-headed Sparrow (<em>Aimophila ruficauda</em>)
Main Article Content
Abstract
Veterinaria México OA
ISSN: 2448-6760
Cite this as:
- Sánchez-Godoy FD, Chávez-Maya F, Méndez-Bernal A, García-Espinosa G, Guerrero-Molina C, Ledesma-Martínez N, et al. Sarcocystis sp. parasites in the Mexican Great-tailed Grackle (Quiscalus mexicanus), Bronzed Cowbird (Molothrus aeneus), and Stripe-headed Sparrow (Aimophila ruficauda). Veterinaria México OA. 2014;1(2). doi: 10.21753/vmoa.1.2.336.
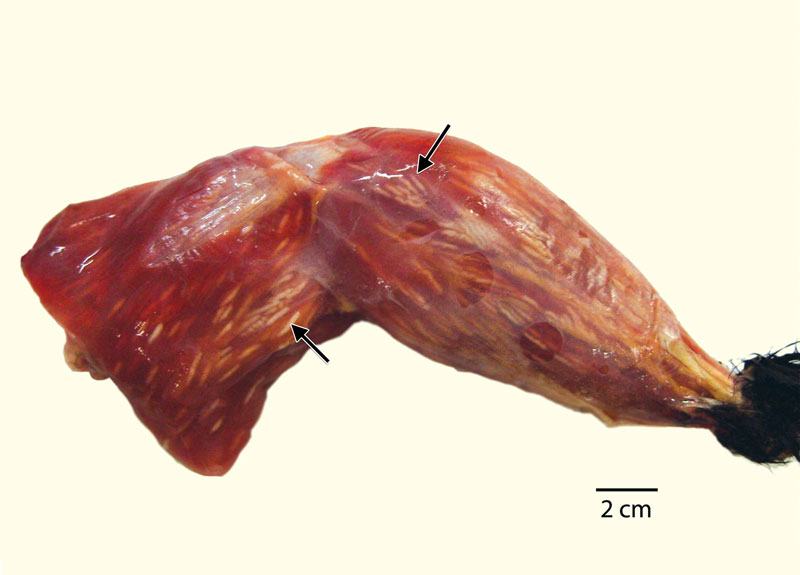
The objective of this study was to describe the morphological and ultraestructural characteristics, the polymerase chain reaction and restriction fragment length polymorphism (PCR-RFLP) results, the sequences and the phylogenetic analysis of a specific fragment of internal transcribed spacer 1 (ITS-1), amplified using the 25/396 primers, of the Sarcocystis sp. parasites identified in the muscles of wild great-tailed grackles, bronzed cowbirds, and stripe-headed sparrows in Mexico. Fifteen birds with sarcocystosis in their skeletal muscles were studied: 7 great-tailed grackles (Quiscalus mexicanus), 6 bronzed cowbirds (Molothrus aeneus), and 2 stripe-headed sparrows (Aimophila ruficauda). Histopathological analysis revealed thin-walled mature parasite cysts. Ultrastructurally, the cyst wall consisted of a granular layer with villar protrusions and numerous microtubules. The bradyzoites measured 4.1 × 1.6 µm, and micronemes appeared in the anterior third of the conoid. For molecular identification, PCR-RFLP was performed using sequences of a specific fragment of internal transcribed spacer 1 (ITS-1) using the primers 25/396 and Hinf I. Hind III did not cut this fragment. The sequencing results indicated a 100% similarity among the Sarcocystis parasites from the three bird species, and a BLAST search revealed 96% sequence similarity with S. neurona. The phylogenetic analysis shows that the sequences studied are topologically distant to those sequences reported for S. neurona in the United States and in South America and are not related to any group previously reported. Although our morphological and molecular analysis data provide strong evidence that S. neurona uses these bird species as intermediate hosts, future molecular studies with additional DNA fragments, combined with biological studies, will ultimately allow us to convincingly identify these parasites. This is the first report of a Sarcocystis sp. parasite in wild birds in Mexico that may be S. neurona.
Article Details
References
1) Bolon B, Greiner EC, Mays MB. 1989. Microscopic features of Sarcocystis falcatula in skeletal muscle from a Patagonian conure. Veterinary Pathology 26:282-284.
2) Box ED, Duszynski DW. 1978. Experimental transmission of Sarcocystis from icterid birds to sparrows and canaries by sporocysts from the opossum. Journal of Parasitology 64:682-688.
3) Box ED, Smith JH. 1982. The intermediate host spectrum in a Sarcocystis species of birds. Journal of Parasitology 68:668-673.
4) Butcher M, Lakritz J, Halaney A, Branson K, Gupta GD, Kreeger J, Marsh AE. 2002. Experimental inoculation of domestic cats (Felis domesticus) with Sarcocystis neurona or S. neurona-like merozoites. Veterinary Pathology 107:1-14.
5) Cheadle MA, Tanhauser SM, Dame JB, Sellon DC, Hines M, Ginn PE, MacKay RJ, Greiner EC. 2001a. The nine-banded armadillo (Dasypus novemcinctus) is an intermediate host for Sarcocystis neurona. International Journal for Parasitology 31:330-335.
6) Cheadle MA, Yowell CA, Sellon DC, Hines M, Ginn PE, Marsh AE, MacKay RJ, Dame JB, Greiner EC. 2001b. The striped skunk (Mephitis mephitis) is an intermediate host for Sarcocystis neurona. International Journal for Parasitology 31:843-849.
7) Dame JB, MacKay RJ, Yowell CA, Cutler TJ, Marsh A, Greiner EC. 1995. Sarcocystis falcatula from passerine and psittacine birds: Synonymy with Sarcocystis neurona, agent of equine protozoal myeloencephalitis. Journal of Parasitology81:930-935.
8) Dubey JP, Lindsay DS. 1998. Isolation in immunodeficient mice of Sarcocystis neurona from opossum (Didelphis virginiana) faeces, and its differentiation from Sarcocystis falcatula. International Journal for Parasitology 28:1823-1828.
9) Dubey JP, Davis SW, Speer CA, Bowman DD, De Lahunta A, Granstrom DE, Topper MJ, Hamir AN, Cummings JF, Suter MM. 1991. Sarcocystis neurona n. sp. (Protozoa: Apicomplexan), the etiologic agent of equine protozoal myeloencephalitis. Journal of Parasitology 77:212-218.
10) Dubey JP, Johnson GC, Bermudez A, Suedmeyer KW, Fritz DL. 2001e. Neural Sarcocystosis in a Straw-necked Ibis (Carphibis spinicollis) associated with a Sarcocystis neurona-like organism and description of muscular Sarcocystis of an unidentified Sarcocystis species. Journal of Parasitology 87:1317-1322.
11) Dubey JP, Lindsay DS. 1999. Sarcocystis speeri n. sp. (Protozoa: Sarcocystidae) from the opossum (Didelphis virginiana). Journal of Parasitology 85:903-909.
12) Dubey JP, Lindsay DS, Rosenthal BM, Kerber CE, Kasai N, Pena HF, Kwok OC, Shen SK, Gennari SM. 2001b. Isolates of Sarcocystis falcatula-like organisms from South American opossums Didelphis marsupialis and Didelphis albiventris from Sao Paulo, Brazil. Journal of Parasitology 87:1449-1453.
13) Dubey JP, Lindsay DS, Saville WJ, Reed SM, Granstrom DE, Speer CA. 2001a. A review of Sarcocystis neurona and equine protozoal myeloencephalitis (EPM). Veterinary Parasitology 95:89-131.
14) Dubey JP, Lindsay DS, Venturini L, Venturini C. 2000. Characterization of Sarcocystis falcatula isolates from the Argentinian opossum, Didelphis albiventris. Journal Eukaryotic Microbiology 47:260-263.
15) Dubey JP, Rosenthal BM, Speer CA. 2001c. Sarcocystis lindsayi n. sp. (Protozoa: Sarcocystidae) from the South American opossum, Didelphis albiventris from Brazil. Journal Eukaryotic Microbiology 48:595-603.
16) Dubey JP, Saville WJ, Stanek JF, Lindsay DS, Rosenthal BM, Oglesbee MJ, Rosypal AC, Njoku CJ, Stich RW, Kwok OC, Shen SK, Hamir AN, Reed SM. 2001d. Sarcocystis neurona infections in raccoons (Procyon lotor): evidence for natural infection with sarcocysts, transmission of infection to opossums (Didelphis virginiana), and experimental induction of neurologic disease in raccoons. Veterinary Parasitology 100:117-129.
17) Dubey JP, Speer CA, Lindsay DS. 1998. Isolation of a third species of Sarcocystis in immunodeficient mice fed feces from opossums (Didelphis virginiana) and its differentiation from Sarcocystis falcatula and Sarcocystis neurona. Journal of Parasitology 84:1158-1164.
18) Dubey JP, Zarnke R, Thomas NJ, Wong SK, Van Bonn W, Briggs M, Davis JW, Ewing R, Mense M, Kwok OC, Romand S, Thulliez P. 2003. Toxoplasma gondii, Neospora caninum, Sarcocystis neurona, and Sarcocystis canis-like infections in marine mammals. Veterinary Parasitology 116:275-296.
19) Elsheikha MH, Murphy JA, Mansfield SL. 2005. Phylogenetic congruence of Sarcocystis neurona Dubey et al, 1991(Apicomplexa: Sarcocystidae) in the United States based on sequence analysis and restriction fragment length polymorphism (RFLP). Systematic Parasitology 61:191-202.
20) Fenger CK, Granstrom DE, Langemeier JL, Stamper S, Donahue JM, Patterson JS, Gajadhar AA, Marteniuk JV, Xiaomin Z, Dubey JP. 1995. Identification of opossums (Didelphis virginiana) as the putative host of Sarcocystis neurona. Journal of Parasitology 81:916-919.
21) Fenger CK, Granstrom DE, Gajadhar AA, Williams NM, McCrillis SA, Stamper S, Langemeier JL, Dubey JP. 1997. Experimental induction of equine protozoal myeloencephalitis in horses using Sarcocystis spp. from the opossum. Veterinary Parasitology 68:199-213.
22) Hayat M. 2000. Principles and techniques of electron microscopy. biological applications. 4th ed. Cambridge, USA: Cambridge University Press.
23) Hillyer EV, Anderson MP, Greiner EC, Atkinson CT, Frenkel JK. 1991. An outbreak of Sarcocystis in a collection of psittacines. Journal of Zoo and Wildlife Medicine 22:434.-445.
24) MacKay RJ, Granstrom DE, Saville WJ, Reed SM. 2000. Equine protozoal myeloencephalitis. Veterinary Clinics of North America: Equine Practice.16:405-425.
25) Mansfield LS, Mehler S, Nelson K, Elsheikha HM, Murphy AJ, Knust B, Tanhauser SM, Gearhart PM, Rossano MG, Bowman DD, Schott HC, Patterson JS. 2008. Brown-headed cowbirds (Molothrus ater) harbor Sarcocystis neurona and act as intermediate host. Veterinary Parasitology 153:24-43.
26) Marsh AE, Barr BC, Lakritz J, Nordhausen R, Madigan JE, Conrad PA. 1997a. Experimental infection of nude mice as a model for Sarcocystis neurona-associated encephalitis. Parasitology Research 83:706-711.
27) Marsh AE, Barr BC, Tell L, Bowman DD, Conrad PA, Ketcherside C, Green T. 1999. Comparison of the internal transcribed spacer, ITS-1, from Sarcocystis falcatula isolates and Sarcocystis neurona. Journal of Parasitology 85:750-757.
28) Marsh AE, Barr BC, Tell L, Koski M, Greiner E, Dame J, Conrad PA. 1997b. In vitro cultivation and experimental inoculation of Sarcocystis falcatula and Sarcocystis neurona merozoitos into budgerigars (Melopsittacus undulatus). Journal of Parasitology 83:1189-1192.
29) Miller MA, Sverlow K, Crosbie PR, Barr BC, Lowenstine LJ, Gulland FM, Packham A, Conrad PA. 2001. Isolation and characterization of two parasitic protozoa from a Pacific harbor seal (Phoca vitulina richardsi) with meningoencephalomyelitis. Journal of Parasitology 87:816-822.
30) Monteiro RM, Keid LB, Richtzenhain LJ, Valadas SY, Muller G, Soares RM. 2013. Extensively variable surface antigens of Sarcocystis spp. infecting Brazilian marsupials in the genus Didelphis occur in myriad allelic combinations, suggesting sexual recombination has aided their diversification. Veterinary Parasitology 196:64-70.
31) Mullaney T, Murphy AJ, Kiupel M, Bell JA, Rossano MG, Mansfield LS. 2005. Evidence to support horses as natural intermediate hosts for Sarcocystis neurona. Veterinary Parasitology 133:27-36.
32) Munday BL, Hartley WJ, Harrigan KE, Presidente PJ, Obendorf DL. 1979. Sarcocystis and related organisms in Australian wildlife: II. Survey findings in birds, reptiles, amphibians and fish. Journal of Wildlife Diseases 15:57-73.
33) Speer CA, Dubey JP. 2001. Ultrastructure of schizonts and merozoites of Sarcocystis neurona. Veterinary Parasitology 95:263-271.
34) Tamura K, Dudley J, Nei M, Kumar S. 2007.. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Molecular Biology and Evolution 24:1596-1599.
35) Tanhauser SM, Yowell CA, Cutler TM, Greiner EC, Mackay RJ, Dame JB. 1999. Multiple DNA markers differentiate Sarcocystis neurona and Sarcocystis falcatula. Journal of Parasitology 85:221-228.
36) Villar D, Kramer M, Howard L, Hammond E, Cray C, Latimer K. 2008. Clinical presentation and pathology of Sarcocystosis in psittaciform birds: 11 cases. Avian Diseases 52:187-194.
37) Yeargan MR, Alvarado-Esquivel C, Dubey JP, Howe DK. 2013. Prevalence of antibodies to Sarcocystis neurona and Neospora hughesi in horses from Mexico. Parasite 20, 29. http://dx.doi.org/10.1051/parasite/2013029 [published: September 10th].
License

Veterinaria México OA by Facultad de Medicina Veterinaria y Zootecnia - Universidad Nacional Autónoma de México is licensed under a Creative Commons Attribution 4.0 International Licence.
Based on a work at http://www.revistas.unam.mx
- All articles in Veterinaria México OA re published under the Creative Commons Attribution 4.0 Unported (CC-BY 4.0). With this license, authors retain copyright but allow any user to share, copy, distribute, transmit, adapt and make commercial use of the work, without needing to provide additional permission as long as appropriate attribution is made to the original author or source.
- By using this license, all Veterinaria México OAarticles meet or exceed all funder and institutional requirements for being considered Open Access.
- Authors cannot use copyrighted material within their article unless that material has also been made available under a similarly liberal license.



