A single neonatal administration of soybean oil and/or tamoxifen affects permanently the testis histomorphology in adult rats.
Main Article Content
Abstract
Veterinaria México OA
ISSN: 2448-6760
Cite this as:
- González González A, González Padilla E, Fierro Fierro F, Juárez Mosqueda M de L, Pérez Rivero JJ, Vergara Onofre M. A single neonatal administration of soybean oil and/or tamoxifen affects permanently the testis histomorphology in adult rats. Veterinaria México OA. 2016;3(2). doi: 10.21753/vmoa.3.2.364
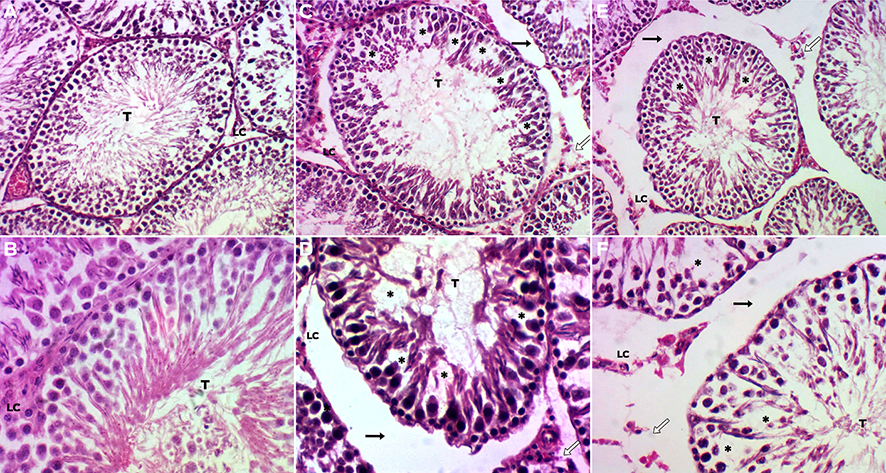
The aim of this study was to determine the effect of tamoxifen (Tx) and its vehicle, soybean oil (SO), during the critical period of hypothalamic sexual differentiation in newborn male rats, regarding gonadal histomorphology during adulthood. The animals were randomly divided into 3 groups (n = 5 each). An hour after birth, one group was treated subcutaneously with 200 μg of Tx, using commercial SO (20 uL) as a vehicle; another group was treated with only 20 μL of SO; the control group received no treatment. All rats were weighed and sacrificed by cervical dislocation on day 90 post-treatment. Testicles were removed, weighed and processed for histological evaluation. The single administration of Tx and/or SO during the critical period of sexual differentiation of the hypothalamus permanently altered testicular histomorphology, spermatogenesis, and body weight in adulthood. Alterations included vacuolization and reduction in the number of spermatogonia and Sertoli cells. The administration of Tx reduced the testicular weight, the diameter and area of the seminiferous tubules, and the height of the germinal epithelium, and increased the intertubular space. Soybean oil by itself reduced the number of spermatocytes and spermatids more than Tx did. There was no effect on the number of Leydig cells. The possibility that soybean oil can act as an endocrine disruptor deserves greater attention and opens the possibility for the development of new methods of pest control.
Article Details
References
Arnold PA. 2009. The organizational-activational hypothesis as the foundation for a unified theory of sexual differentiation of all mammalian. Hormones and Behavior, 55(5):570-578.
Herrera GH, Rosado GA, Vergara OM, Salcedo VM, Miliar GA, Heuze de IY, et al. 2013. Genetic expression associated to cell cycle, apoptosis, synaptogenesis and cell differentiation during sex differentiation in rats. Revista Mexicana de Ciencias Pecuarias, 4(3):289-304.
Simerly R. 2002. Wired for reproduction: Organization and development of sexually dimorphic circuits in the mammalian forebrain. Annual Review of Neuroscience, 25:507-536. .
Babichev V, Shishkina I, Peryshkova T. 1990. The effect of neonatal castration of male rats on the level of sex-hormone receptors in the hypothalamus and hypophysis of adult animals. Biomedical Science, 1:189-192.
Finkelstein JS, O’Dea LSL, Whitcomb RW, Crowley WF. 1991. Sex steroid control of gonadotropin secretion in the human male. II Effects of estradiol administered in normal and gonadotropin-releasing hormone-deficient men. Journal of clinical Endocrinology and Metabolism, 70:621-628.
Bagatell CJ, Dahl KD, Bremmer WJ. 1994. The direct pituitary effect of testosterone to inhibit gonadotropin secretion in men is partially mediated by aromatization to estradiol. Journal of Andrology, 15:15-21.
Lauber ME, Sarasin A, Lichtensteiger W. 1997. Sex differences and androgen-dependent regulation of aromatase (CYP19) mRNA expression in the developing and adult rat brain. Journal of Steroid Biochemistry and Molecular Biology, 61:359-364.
Balasinor N, Parte P, Gill-Sharma MK, Juneja HS. 2001. Effect of tamoxifen on sperm fertilising ability and preimplantation embryo development. Molecular and Cellular Endocrinology,178:199-206.
Barraclough A. 1967. Modifications in reproductive function after exposure to hormones during the prenatal and early posnatal period. In: Martini L, Ganong WF, editors. Neuroendocrinology. New York and London Academic Press; p. 61-99.
Dörner G, Staudt J. 1968. Structural changes in the preoptic anterior hypothalamic area of the male rat. Following neonatal castration and androgen treatment. Neuroendocrinology, 3:136-140.
Döhler K, Srivastava S, Shryne J, Jarzab B, Sipos A, Gorski R. 1984. Differentiation of the sexually dimorphic nucleus in the preoptic area of the rat brain is inhibited by posnatal treatment with an estrogen antagonist. Neuroendocrinology, 38:297-301.
Gill-Sharma MK, Gopalkrishnan K, Balasinor N, Parte P, Jayaraman S, Juneja HS. 1993. Effects of tamoxifen on the fertility of male rats. Journal of Reproduction and Fertility, 99:395-402.
Furr BJA, Jordan VC. 1984. The pharmacology and clinical uses of tamoxifen. Pharmacology and Therapeutics, 25:127-205.
Macnab MW, Tallarida RJ, Joseph R. 1984. An evaluation of tamoxifen as a partial agonist by classical receptor theory — an explanation of the dual action of tamoxifen. European Journal of Pharmacology, 103:321-326.
Iguchi T, Hirokawa M. 1986. Changes in the male genital organs of mice exposed neonatally to tamoxifen. Proceedings of the Japan Academy, 62:157-160.
Taguchi N. 1987. Reproductive tract lesions in the male mice treated neonatally with tamoxifen. Biology of Reproduction, 37:113-116.
Döhler KD, Hines M, Coquelin A, Davis F, Shryne JE, Sickmöler PM, et al. 1985. Pre- and posnatal influence of an estrogen antagonist and an androgen antagonist on differentiation of the preoptic area in male and female rats. Neuroendocrinology, 42:443-448.
Wilson CA, Davies DC. 2007. The control of sexual differentiation of the reproductive system and brain. Reproduction, 133:331-359.
Roberts D, Veeramachaneni DN, Schlaff WD, Awoniyi CA. 2000. Effects of chronic dietary exposure to genistein, a phytoestrogen, during various stages of development on reproductive hormones and spermatogenesis in rats. Endocrine, 13(3):281–286.
Wisniewski AB, Klein SL, Lakshmanan Y, Gearhart JP. 2003. Exposure to genistein during gestation and lactation demasculinizes the reproductive system in rats. Journal of Urology, 169(4):1582– 1586.
Levy JR, Faber KA, Ayyash L, Hughes CL Jr. 1995. The effect of prenatal exposure to the phytoestrogen genistein on sexual differentiation in rats. Proceedings of the Society for Experimental Biology and Medicine, 208(1):60-66.
Wisniewski AB, Cernetich A, Gearhart JP, Klein SL. 2005. Perinatal exposure to genistein alters reproductive development and aggressive behaviour in male mice. Physiology and Behavior; 84:327-334.
Herrera G, Vergara O, Rosado GA, Rosales T. 2005. Sexual differentiation in the central nervous system. Veterinaria México, 36:339-360.
Clermont, Y. 1972. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiological Reviews. 52, 198–236.
Berndtson, WE. 1977. Methods for quantifying mammalian spermatogenesis: a review. Journal of Animal Science, 44, 818–833.
Hess, RA., LR Franca. 2007. Spermatogenesis and cycle of the seminiferous epithelium. In: Molecular Mechanism in Spermatogenesis. (C. Y. Cheng, ed). Urbana, IL: Landes Bioscience and Springer Science + Business Media. pp. 1–15.
Farias TO., Notini AA, Talamoni SA, Godinho HP. 2014. Testis Morphometry and Stages of the Seminiferous Epithelium Cycle in an Epididymal Sperm-storing Neotropical Vespertilionid, Myotis levis (Chiroptera). Journal of Veterinary Medicine, 44;361–369.
Huang B, Harper DAT, Hammer O. 2013. Introduction to PAST, A Comprehensive Statistics Software Package for Paleontological Data Analysis. Acta Paleontologica Sinica, 52(2):181.
Carreau S, Delalande C, Silandre D, Bourguiba S, Lambard S. 2006. Aromatase and estrogen receptors in male reproduction. Molecular and Cellular Endocrinology, 246; 65–68.
Carreau S, Genissel C, Bilinska B, Levallet J. 1999. The oestrogen sources in the testis and the reproductive tract of the male. International Journal of Andrology, 22:211-213.
Carreau S, Lambard S, Delalande C, Denis GI, Bilinska B, Bourguiva S. 2003. Aromatase expression and role of estrogens in male gonad: a review. Reproductive Biology and Endocrinology, 35:1-6.
Assinder S, Davis R, Fenwick M, Glover A. 2007. Adult-only exposure of male rats to a diet of high phytoestrogens content increase apoptosis of meiotic and post-meiotic germ cells. Reproduction, 133:11-19.
Pfeiffer CA. 1936. Sexual differences of the hypophysis and their determination by the gonads. American Journal of Anatomy, 58:195-225.
Phoenix CH, Goy RW, Gerall AA, Young WC. 1959. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology, 65(3):369-382.
Barraclough CA. 1966. Modifications in the CNS regulation of reproduction after exposure of prepubertal rats to steroid hormones. Recent Progress in Hormone Research Journal, 22:503-539.
Bellido C, Martín de las Mulas J, Tena-Sempere M, Aguilar R, Alonso R, Sánchez-Criado JE. 2003. Tamoxifen induces gonadotropin-releasing hormone self-priming through an estrogen-dependent progesterone receptor expression in the gonadotrope of the rat. Neuroendocrinology, 77(6):425-435.
Nazarewicz RR, Zenebe WJ, Parihar A, Larson SK, Alidema E, Choi J, et al. 2007. Tamoxifen Induces Oxidative Stress and Mitochondrial Apoptosis via Stimulating Mitochondrial Nitric Oxide Synthase. Cancer Research, 67(3):1282-1290.
Padmalatha RS, Vijayalaxmi KK. 2001. Tamoxifen citrate induced sperm shape abnormalities in the in vivo mouse. Mutation Research, 492:1–6.
D'Souza UJA. 2003. Tamoxifen induced multinucleated cells (symplasts) and distortion of seminiferous tubules in rat testis. Asian Journal of Andrology, 5(3): 217-220.
Balasinor N, Gill-Sharma MK, Parte P, D’Souza S, Kedia N, Juneja HS. 2002. Effect of paternal administration of an antiestrogen, tamoxifen on embryo development in rats. Molecular and Cellular Endocrinology, 190:159-166.
Sunghak L, Myeong-Seop L, Joonghoon P, Jin YZ, Dong IJ. 2012. Oxidative stress in the testis induced by tamoxifen and its effects on early embryo development in isogenic mice. Journal of Toxicology Science, 37(4):675-679.
Cederroth CR, Zimmermann C, Nef SS. 2012. Phytoestrogens and their impact on reproductive health. Molecular and Cellular Endocrinology, 355:192-200.
Abney TO. 1999. The potential roles of estrogens in regulating Leydig cell development and function: A review. Steroids, 64:610-617.
Hess RA, Bunick D, Lubahn BD, Zhou Q, And Bouma J. 2000. Morphologic Changes in Efferent Ductules and Epididymis in Estrogen Receptor-a Knockout Mice. Journal of Andrology , 21(1):107-121.
Hess RA, Bunick D, Bahr J. 2001. Oestrogen its receptor and function in male reproductive tract- a review. Molecular and Cellular Endocrinology, 178:29-38.
Saunders PTK. 2005. Does estrogen receptor b play a significant role in human reproduction?. TRENDS in Endocrinology and Metabolism, 16(5):222-227.
Lambard S, Silandre D, Delalande C, Galeraud DI, Bourguiba S, Carreau S. 2005. Aromatase in testis: Expression and role in male reproduction. Journal of Steroid Biochemistry & Molecular Biology, 95:63-9.
Carreau S, Bourguiba S, Lambard S, Galeraud DI, Genissel C, Levallet J. 2002. Reproductive system: aromatase and estrogens. Molecular and Cellular Endocrinology, 193:137-143.
Scobie GA, Macpherson S, Millar MR, Groome NP, Romana P, Saunders PTK. 2002. Human estrogen receptors: differential expression of ERalpha and beta and the identification of ERbeta variants. Steroids, 67:985-992.
Perez RJJ, Martinez MJJ, Perez MM, Aguilar SA, Garcia SMD Serrano H. 2009. Phytoestrogen treatment induces testis alterations in dogs. Potential use in population control. Veterinary Research Communications, 33:87-95.
Pérez RJ.J., Pérez MM & Aguilar SA. 2014. Histometric analysis of vampire bat (Desmodus rotundus) testicles treated with coumestrol by oral route, Journal of Applied Animal Research, 42(2): 208-212.
Yu-hua L, Fei D, Fen Y, Xiao-Yu Z, Hong-jie P, Yang L, et al. 2015. Pubertal exposure to bisphenol A affects the reproduction of male mice and sex ratio of offspring. Journal of Reproduction & Contraception, 26(1):14-21.
Kosif R, Yilmaz F, Evrendilek AG, Diramali M. 2010. Histopathological Effects of Aloe barbadensis and Soybean Oil on Rat Liver. International Journal of Morphology, 28(4):1101-1106.
Wathes DC, Abayasekara DR, Aitken RJ. 2007. Polyunsaturated fatty acids in male and female reproduction. Biology of Reproduction, 77:190-201.
Gurr MI, Harwood JL, Frayn KN. 2002. Lipid Biochemistry: An Introduction. 5th ed. Oxford, UK: Blackwell Science Ltd.
Allen KGD, Harris MA. 2001. The role of n-3 fatty acids in gestation and parturition. Experimental Biology and Medicine, 226:498–506.
Simopoulos AP. 2002. Omega-3 fatty acids in inflammation and autoimmune diseases. Journal of American College of Nutrition, 21:495–505.
License

Veterinaria México OA by Facultad de Medicina Veterinaria y Zootecnia - Universidad Nacional Autónoma de México is licensed under a Creative Commons Attribution 4.0 International Licence.
Based on a work at http://www.revistas.unam.mx
- All articles in Veterinaria México OA re published under the Creative Commons Attribution 4.0 Unported (CC-BY 4.0). With this license, authors retain copyright but allow any user to share, copy, distribute, transmit, adapt and make commercial use of the work, without needing to provide additional permission as long as appropriate attribution is made to the original author or source.
- By using this license, all Veterinaria México OAarticles meet or exceed all funder and institutional requirements for being considered Open Access.
- Authors cannot use copyrighted material within their article unless that material has also been made available under a similarly liberal license.



