Ovicidal activity of extracts from four plant species against the cattle nematode <em>Cooperia punctata</em>
Main Article Content
Abstract
Veterinaria México OA
ISSN: 2448-6760
Cite this as:
- von Son de Fernex E, Alonso Díaz MÁ, Mendoza de Gives P, Valles de la Mora B, Zamilpa A, González Cortasar M. Ovicidal activity of extracts from four plant species against the cattle nematode Cooperia punctata. Veterinaria México OA. 2016;3(2). doi: 10.21753/vmoa.3.2.365.
Bioactive plants might represent an alternative for Cooperia punctata control in grazing cattle. The objectives of this study were (1) to assess the ovicidal activity of extracts from 4 plant species against C. punctata, (2) to determine the role of the polyphenols in the plants’ anthelmintic (AH) activity, and (3) to evaluate the best plant extraction procedure when searching for ovicidal activity. The egg hatch assay was used with different extraction procedures, aqueous (AQ), acetone:water (AW) and acetonic (AC), to evaluate the ovicidal activity of Leucaena leucocephala, Gliricidia sepium, Guazuma ulmifolia and Cratylia argentea. Eggs of C. punctata were exposed in quadruplicate to 0.6, 1.2, 2.4, 4.8 and 9.6 mg mL-1 of each plant extract. The roles of the polyphenols were assessed using polyethylene glycol (PEG). The 12 plant extracts inhibited egg hatching in a dose-dependent manner. Best-fit LC50 values were 1.03 ± 0.17 and 7.90 ± 1.19 mg mL-1 for G. sepium-AC and L. leucocephala-AQ, respectively. Differences in AH activity were found among the extraction procedures (P < 0.05). At the highest concentration, L. leucocephala-AQ inhibited more than 50% of C. punctata hatching. The G. sepium-AC extract fully inhibited egg hatching. The addition of polyethylene glycol revealed the role of the polyphenols in the bioactivity of most plant extracts; however, for G. sepium-AC, the polyphenols were not the main bioactive compounds. Overall, acetone:water extraction represented the best extraction procedure to obtain both ovicidal activity and higher yield. The inhibition rates suggested that L. leucocephala and G. sepium should be evaluated as a means of reducing larval density in pastures.
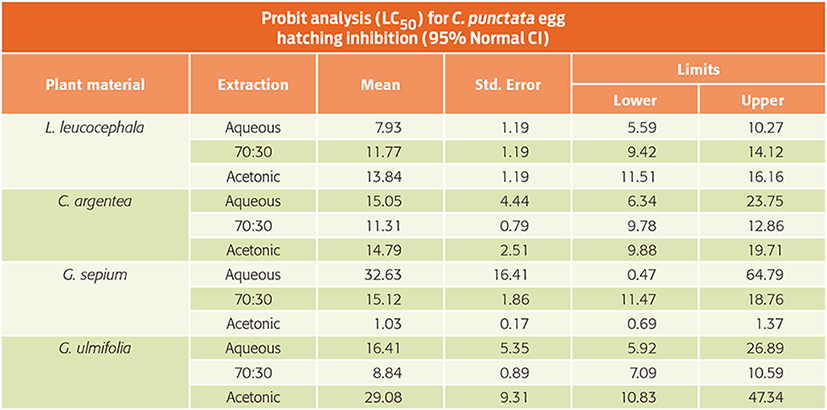
Table 1. Lethal concentrations required to inhibit 50% of Cooperia punctata egg hatching (LC50), after a 48-h incubation period with bioactive extracts (mg mL-1).
Article Details
References
Alonso-Diaz MA, Torres-Acosta JF, Sandoval-Castro, CA, Aguilar-Caballero, A.J., Hoste, H. 2008a. In vitro larval migration and kinetics of exsheathment of Haemonchus contortus larvae exposed to four tropical tanniniferous plant extracts. Veterinary Parasitology, 153:313-319.
Alonso-Diaz MA, Torres-Acosta JF, Sandoval-Castro CA, Capetillo-Leal C, Brunet S, Hoste H. 2008b. Effects of four tropical tanniniferous plant extracts on the inhibition of larval migration and the exsheathment process of Trichostrongylus colubriformis infective stage. Veterinary Parasitology, 153:187-192.
Alonso-Diaz MA, Torres-Acosta JF, Sandoval-Castro CA, Hoste H. 2010. Tannins in tropical tree fodders fed to small ruminants: A friendly foe? Small Ruminant Research, 89:164-173.
Arnaud-Ochoa R, Alonso Díaz MA. 2012. Unidades de producción bovina con nematodos gastrointestinales resistentes al albendazol (benzimidazoles) en México. Revista Cientifica, XXII (4):315-320.
Athanasiadou S, Kyriazakis I, Jackson F, Coop RL. 2001. Direct anthelmintic effects of condensed tannins towards different gastrointestinal nematodes of sheep in vitro and in vivo studies. Veterinary Parasitology, 99:205-219.
Barrau E, Fabre N, Fouraste I, Hoste H. 2005. Effect of bioactive compounds from Sainfoin (Onobrychis viciifolia Scop.) on the in vitro larval migration of Haemonchus contortus: Role of tannins and flavonol glycosides. Parasitology, 131:531-538.
Bartley DJ, McArthur CL, Devin LM, Sutra JF, Morrison AA, Lespine A, Matthews JB. 2012. Characterisation of macrocyclic lactone resistance in two field-derived isolates of Cooperia oncophora. Veterinary Parasitology, 190:454-460.
Becerra-Nava R, Alonso-Diaz MA, Fernandez-Salas A, Quiroz RH. 2014. First report of cattle farms with gastrointestinal nematodes resistant to levamisole in Mexico. Veterinary Parasitology, 204:285-290.
Biavatti MW. 2009. Synergy: An old wisdom, a new paradigm for pharmacotherapy. Brazilian Journal of Pharmaceutical Sciences, 45:371-378.
Bizimenyera ES, Githiori JB, Eloff JN, Swan GE. 2006. In vitro activity of Peltophorum africanum Sond. (Fabaceae) extracts on the egg hatching and larval development of the parasitic nematode Trichostrongylus colubriformis. Veterinary Parasitology, 142:336-343.
Botura MB, dos Santos JD, da Silva GD, de Lima HG, de Oliveira JV, de Almeida MA, Batatinha MJ, Branco A. 2013. In vitro ovicidal and larvicidal activity of Agave sisalana Perr. (sisal) on gastrointestinal nematodes of goats. Veterinary Parasitology, 192:211-217.
Charlier J, Hoglund J, von Samson-Himmelstjerna G, Dorny P, Vercruysse J. 2009. Gastrointestinal nematode infections in adult dairy cattle: Impact on production, diagnosis and control. Veterinary Parasitology, 164:70-79.
Chavan UD, Amarowicz R. 2013. Effect of various solvent systems on extraction of phenolics, tannins and sugars from beach pea (Lathyrus maritimus L.). International Food Research Journal, 20(3):1139-44.
Chavan UD, Shahidia F, Naczkb M. 2001. Extraction of condensed tannins from beach pea (Lathyrus maritimus L.) as affected by different solvents. Food Chemistry, 75:509–512.
Coles GC, Bauer C, Borgsteede FHM, Geerts S, Klei TR, Taylor MA, Waller PJ. 1992. World Association for the Advancement of Veterinary Parasitology (WAAVP) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Veterinary Parasitology, 44:35-44.
Demeler J, Krucken J, AlGusbi S, Ramunke S, De Graef J, Kerboeuf D, Geldhof P, Pomroy WE, von Samson-Himmelstjerna G. 2013. Potential contribution of P-glycoproteins to macrocyclic lactone resistance in the cattle parasitic nematode Cooperia oncophora. Molecular & Biochemical Parasitology, 188:10-19.
Dobson RJ, Donald AD, Waller PJ, Snowdown KL. 1986. An egg-hatch assay for resistance to levamisole in trichostrongyloid nematode parasites. Veterinary Parasitology, 19:77-84.
Fitzpatrick JL. 2013. Global food security: The impact of veterinary parasites and parasitologists. Veterinary Parasitology, 195:233-248.
Flores-Guido JS. 2001. Leguminosae. Florística, Etnobotánica y Ecología. Etnoflora Yucatense. Universidad Autónoma de Yucatán; Mérida, Yucatán, México.
Gasbarre LC. 2014. Anthelmintic resistance in cattle nematodes in the US. Veterinary Parasitology, 204:3-11.
Gibbons LM. 1981. Revision of the african species of the genus Cooperia Ransom, 1907 (Nematoda, trichostrongylidae). Systematic Parasitology, 2:219-252
Hoste H, Martinez-Ortiz-De-Montellano C, Manolaraki F, Brunet S, Ojeda-Robertos N, Fourquaux I, Torres-Acosta JF, Sandoval-Castro CA. 2012. Direct and indirect effects of bioactive tannin-rich tropical and temperate legumes against nematode infections. Veterinary Parasitology, 186:18-27.
Katiki LM, Chagas AC, Bizzo HR, Ferreira JF, Amarante AF. 2011. Anthelmintic activity of Cymbopogon martinii, Cymbopogon schoenanthus and Mentha piperita essential oils evaluated in four different in vitro tests. Veterinary Parasitology, 183:103-108.
Kenyon F, Jackson F. 2012. Targeted flock/herd and individual ruminant treatment approaches. Veterinary Parasitology, 186:10-17.
Kozan E, Anul SA, Tatli II. 2013. In vitro anthelmintic effect of Vicia pannonica var. purpurascens on trichostrongylosis in sheep. Experimental Parasitology, 134:299-303.
Makkar HPS. 2003. Quantification of tannins in tree and shrub foliage. A laboratory manual. Dordrecht, Netherlands: Kluwer Academic Publishers.
Makkar HPS, Blummel M, Becker K. 1995. Formation of complexes between polyvinyl pyrrolidones or polyethylene glycols and tannins, and their implication in gas production and true digestibility in vitro techniques. British Journal of Nutrition, 73:897-913.
Mansfield LS, Gamble HR, Fetterer RH. 1992. Characterization of the eggshell of haemonchus contortus-i. Structural components. Comparative Biochememistry and Physiology, 103B(3):681-686.
Martinez-Ortiz-de-Montellano C, Vargas-Magana JJ, Canul-Ku HL, Miranda-Soberanis R, Capetillo-Leal C, Sandoval-Castro CA, Hoste H, Torres-Acosta JF. 2010. Effect of a tropical tannin-rich plant Lysiloma latisiliquum on adult populations of Haemonchus contortus in sheep. Veterinary Parasitology, 172:283-290.
Molan AL, Duncan AJ, Barry TN, McNabb WC. 2003. Effects of condensed tannins and crude sesquiterpene lactones extracted from chicory on the motility of larvae of deer lungworm and gastrointestinal nematodes. Parasitology International, 52:209-218.
Molan AL, Waghorn GC, Min BR, McNabb WC. 2000. The effect of condensed tannins from seven herbages on Trichostrongylus colubriformis larval migration in vitro. Folia Parasitologica, 47:39-44.
Novobilsky A, Mueller-Harvey I, Thamsborg SM. 2011. Condensed tannins act against cattle nematodes. Veterinary Parasitology, 182:213-220.
Perri AF, Mejia ME, Licoff N, Lazaro L, Miglierina M, Ornstein A, Becu-Villalobos D, Lacau-Mengido IM. 2011. Gastrointestinal parasites presence during the peripartum decreases total milk production in grazing dairy Holstein cows. Veterinary Parasitology, 178:311-318.
Rafi M, Rohaeti E, Miftahudin A, Darusman LK. 2011. Differentiation of Curcuma longa, Curcuma xanthorrhiza and Zingiber cassumunar by thin layer chromatography fingerprint analysis. Indonesian Journal of Chemistry, 11(1):71-74.
Stromberg BE, Gasbarre LC, Waite A, Bechtol DT, Brown MS, Robinson NA, Olson EJ, Newcomb H. 2012. Cooperia punctata: Effect on cattle productivity? Veterinary Parasitology, 183:284-291.
Sutherland IA, Leathwick DM. 2011. Anthelmintic resistance in nematode parasites of cattle: A global issue? Trends in Parasitology, 27:176-181.
Von Son-de Fernex E, Alonso-Diaz MA, Valles-de la Mora B, Capetillo-Leal CM. 2012. In vitro anthelmintic activity of five tropical legumes on the exsheathment and motility of Haemonchus contortus infective larvae. Experimental Parasitology, 131:413-418.
Wabo Poné J, Kenne Tameli F, Mpoame M, Pamo Tedonkeng E, Bilong Bilong C. 2011. In vitro activities of acetonic extracts from leaves of three forage legumes (Calliandra calotyrsus, Gliricidia sepium and Leucaena diversifolia) on Haemonchus contortus. Asian Pacific Journal of Tropical Medicine, 4:125-1 28.
License

Veterinaria México OA by Facultad de Medicina Veterinaria y Zootecnia - Universidad Nacional Autónoma de México is licensed under a Creative Commons Attribution 4.0 International Licence.
Based on a work at http://www.revistas.unam.mx
- All articles in Veterinaria México OA re published under the Creative Commons Attribution 4.0 Unported (CC-BY 4.0). With this license, authors retain copyright but allow any user to share, copy, distribute, transmit, adapt and make commercial use of the work, without needing to provide additional permission as long as appropriate attribution is made to the original author or source.
- By using this license, all Veterinaria México OAarticles meet or exceed all funder and institutional requirements for being considered Open Access.
- Authors cannot use copyrighted material within their article unless that material has also been made available under a similarly liberal license.



