Geographic distribution of <em>Desmodus rotundus</em> in Mexico under current and future climate change scenarios: Implications for bovine paralytic rabies infection
Main Article Content
Abstract
Veterinaria México OA
ISSN: 2448-6760
Cite this as:
- Zarza H, Martínez-Meyer E, Suzán G, Ceballos G. Geographic distribution of Desmodus rotundus in Mexico under current and future climate change scenarios: Implications for bovine paralytic rabies infection. Veterinaria México OA. 2017;4(3). doi: 10.21753/vmoa.4.3.390.
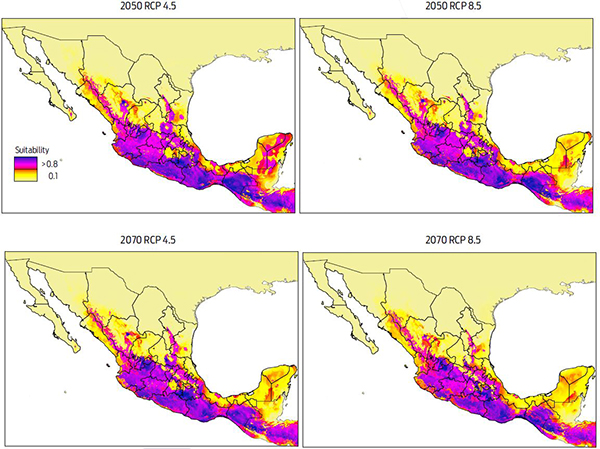
Climate change may modify the spatial distribution of reservoirs hosting emerging and reemerging zoonotic pathogens, and forecasting these changes is essential for developing prevention and adaptation strategies. The most important reservoir of bovine paralytic rabies in tropical countries, is the vampire bat (Desmodus rotundus). In Mexico, the cattle industry loses more than $2.6 million US dollar, annually to this infectious disease. Therefore, we predicted the change in the distribution of D. rotundus due to future climate change scenarios, and examined the likely effect that the change in its distribution will have on paralytic rabies infections in Mexico. We used the correlative maximum entropy based model algorithm to predict the potential distribution of D. rotundus. Consistent with the literature, our results showed that temperature was the variable most highly associated with the current distribution of vampire bats. The highest concentration of bovine rabies was in Central and Southeastern Mexico, regions that also have high cattle population densities. Furthermore, our climatic envelope models predicted that by 2050–2070, D. rotundus will lose 20 % of its current distribution while the northern and central regions of Mexico will become suitable habitats for D. rotundus. Together, our study provides an advanced notice of the likely change in spatial patterns of D. rotundus and bovine paralytic rabies, and presents an important tool for strengthening the National Epidemiological Surveillance System and Monitoring programmes, useful for establishing holistic, long-term strategies to control this disease in Mexico.
Article Details
References
Acha P, Szyfres B. Zoonosis y enfermedades transmisibles comunes al hombre y a los animales: clamidiosis, rickettsiosis y virosis. 3rd ed. Lima (PE): Organización Panemerica de la Salud; 2003.
Johnson N, Vos A, Freuling C, Tordo N, Fooks AR, Muller T. Human rabies due to lyssavirus infection of bat origin. Vet Microbiol. 2010;142(3-4):151-9. doi: 10.1016/j.vetmic.2010.02.001.
Velasco-Villa A, Orciari LA, Juarez-Islas V, Gomez-Sierra M, Padilla-Medina I, Flisser A, et al. Molecular diversity of rabies viruses associated with bats in Mexico and other countries of the Americas. J Clin Microbiol. 2006;44(5):1697-710. doi: 10.1128/JCM.44.5.1697-1710.2006.
Constantine DG. Bat rabies and other lyssavirus infections. Reston VA (US): U. S. Geological Survey; 2009.
Heymann DL. El control de las enfermedades transmisibles. 18th ed. Washington DC (USA): Organización Panemericana de la Salud; 2005.
World Health Organization. Rabia. Nota descriptiva N° 99; 2016.
Belotto A, Leanes LF, Schneider MC, Tamayo H, Correa E. Overview of rabies in the Americas. Virus Res. 2005;111(1):5-12. doi: 10.1016/j.virusres.2005.03.006.
Lee DN, Papes M, Van den Bussche RA. Present and potential future distribution of common vampire bats in the Americas and the associated risk to cattle. PLoS One. 2012;7(8):e42466. doi: 10.1371/journal.pone.0042466.
Schneider MC, Romijn PC, Uieda W, Tamayo H, da Silva DF, Belotto A, et al. Rabies transmitted by vampire bats to humans. REv Panam Salud Pública. 2009;25(3):260-9. doi: 10.1590/S1020-49892009000300010.
Organización Panamericana de la Salud / Organización Mundial de Salud. Vigilancia epidemiológica de la rabia en las Américas. Rio de Janeiro (BR)2004.
Anderson A, Shwiff S, Gebhardt K, Ramirez AJ, Shwiff S, Kohler D, et al. Economic evaluation of vampire bat (Desmodus rotundus) rabies prevention in Mexico. Transboundary and Emerging Diseases. 2012;61(2):140-6. doi: 10.1111/tbed.12007.
Bárcenas-Reyes I, Loza-Rubio E, Zendejas-Martínez H, Luna-Soria H, Cantó-Alarcón GJ, Milián-Suazo F. Comportamiento epidemiológico de la rabia paralítica bovina en la región central de México, 2001–2013. Revista Panamericana de la Salud Publica. 2015;38(5).
Servicio Nacional de Sanidad Inocuidad y Calidad Agroalimentaria. Campaña Nacional para la prevención y control de la rabia en bovinos y especies ganaderas. Mexico.2016 [Available from: https://www.gob.mx/senasica/acciones-y-programas/campana-nacional-para-la-prevencion-y-control-de-la-rabia-en-bovinos-y-especies-ganaderas].
Greenhall AM, Joermann G, Schmidt U. Desmodus rotundus. Mammalian Species. 1983;202:1-6.
Barquez R, Pérez S, Miller B, Díaz M. Desmodus rotundus: IUCN: International Union for Conservation of Nature; 2012 [Available from: www.iucn.org].
Greenhall AM, Schmidt U. Natural history of vampire bats. Florida: CRC Press, Inc.; 1988.
Ávila-Flores R, Medellín RA. Ecological, taxonomic, and physiological correlates of cave use by mexican bats. J Mammal. 2004;85:675-87
Villa-R B. Los murciélagos de México. Mexico City (MX): Universidad Nacional Autónoma de México; 1966.
George DB, Webb CT, Farnsworth ML, O'Shea TJ, Bowen RA, Smith DL, et al. Host and viral ecology determine bat rabies seasonality and maintenance. Proc Natl Acad Sci U S A. 2011;108(25):10208-13. doi: 10.1073/pnas.1010875108.
Peterson AT, Soberón J, Pearson RG, Anderson RP, Martínez-Meyer E, Nakamura M, et al. Ecological niches and geographic distributions. Princeton, New Jersey (US): Princeton University Press; 2011.
Soberon J. Grinnellian and Eltonian niches and geographic distributions of species. Ecol Lett. 2007;10(12):1115-23. doi: 10.1111/j.1461-0248.2007.01107.x.
Guisan A, Tingley R, Baumgartner JB, Naujokaitis-Lewis I, Sutcliffe PR, Tulloch AI, et al. Predicting species distributions for conservation decisions. Ecol Lett. 2013;16(12):1424-35. doi: 10.1111/ele.12189.
Hutchinson GE. An introduction to population ecology. New Haven (US): Yale University Press; 1978. p. 271.
Grinnell J. The niche-relationships of the California Thrasher. The Auk. 1917;34:427-33.
Elton C. Animal Ecology. First ed. New York: Macmillan Company; 1927. p. 260.
GBIF Occurrence Download [Internet]. 2017 [cited 19 May]. Available from: http://www.gbif.org/.
México: Informes semanales sobre enfermedades y plagas de reporte obligatorio inmediato. [Internet]. 2016 [cited 8 February 2015]. Available from: http://www.gob.mx/senasica/acciones-y-programas/sistema-nacional-de-vigilancia-epidemiologica-sive.
Censo agropecuario 2007. VIII Censo agrícola, ganadero y forestal [Internet]. 2009. Available from: http://www.inegi.org.mx/est/contenidos/proyectos/Agro/ca2007/Resultados_Agricola/default.aspx.
Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25(15):1965-78. doi: 10.1002/joc.1276.
Instituto Nacional de Estadística y Geografía. Modelo Digital de Elevación. Mexico (MX).2009 [Available from: http://www.inegi.org.mx/geo/contenidos/datosrelieve/continental/].
Environmental Systems Research Institute. ArcGIS. 9.3 ed. California2009.
Pachauri RK, Meyer LA. Climate change 2014: Synthesis report. Contribution of working groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change. Geneva (CH): Intergovernmental Panel on Climate Change; 2014.
Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecological Modelling. 2006;190(3-4):231-59. doi: 10.1016/j.ecolmodel.2005.03.026.
Phillips SJ, Dudík M. Modeling of species distributions with Maxent: new extensions and a comprehensive evaluation. Ecography. 2008;31:161-75. doi: 10.1111/j.2007.0906-7590.05203.x.
Elith J, Graham CH, Anderson RP, Dudík M, Ferrier S, Guisan A, et al. Novel methods improve prediction of species’ distributions from occurrence data. Ecography. 2006;29:129-51.
Elith J, Phillips SJ, Hastie T, Dudík M, Chee YE, Yates CJ. A statistical explanation of MaxEnt for ecologists. Diversity and Distributions. 2011;17(1):43-57. doi: 10.1111/j.1472-4642.2010.00725.x.
Santika T. Assessing the effect of prevalence on the predictive performance of species distribution models using simulated data. Global Ecology and Biogeography. 2011;20(1):181-92. doi: 10.1111/j.1466-8238.2010.00581.x.
Muscarella R, Galante PJ, Soley-Guardia M, Boria RA, Kass JM, Uriarte M, et al. ENMeval: An R package for conducting spatially independent evaluations and estimating optimal model complexity forMaxentecological niche models. Methods in Ecology and Evolution. 2014;5(11):1198-205. doi: 10.1111/2041-210x.12261.
Lobo JM, Jiménez-Valverde A, Real R. AUC: a misleading measure of the performance of predictive distribution models. Global Ecology and Biogeography. 2007;17:145-51.
Lobo JM, Tognelli MF. Exploring the effects of quantity and location of pseudo-absences and sampling biases on the performance of distribution models with limited point occurrence data. Journal for Nature Conservation. 2011;19(1):1-7. doi: 10.1016/j.jnc.2010.03.002.
Marino J, Bennett M, Cossios D, Iriarte A, Lucherini M, Pliscoff P, et al. Bioclimatic constraints to Andean cat distribution: a modelling application for rare species. Diversity and Distributions. 2011;17(2):311-22. doi: 10.1111/j.1472-4642.2011.00744.x.
Jiménez-Valverde A, Lobo JM. Threshold criteria for conversion of probability of species presence to either–or presence–absence. Acta Oecologica. 2007;31(3):361-9. doi: 10.1016/j.actao.2007.02.001.
Barve N, Barve V, Jiménez-Valverde A, Lira-Noriega A, Maher SP, Peterson AT, et al. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecological Modelling. 2011;222(11):1810-9. doi: 10.1016/j.ecolmodel.2011.02.011.
de Andrade FA, Gomes MN, Uieda W, Begot AL, Ramos Ode S, Fernandes ME. Geographical analysis for detecting high-risk areas for bovine/human rabies transmitted by the common hematophagous bat in the amazon region, Brazil. PLoS One. 2016;11(7):e0157332. doi: 10.1371/journal.pone.0157332.
de Thoisy B, Bourhy H, Delaval M, Pontier D, Dacheux L, Darcissac E, et al. Bioecological drivers of rabies virus circulation in a neotropical bat community. PLoS Negl Trop Dis. 2016;10(1):e0004378. doi: 10.1371/journal.pntd.0004378.
Delpietro HA, Marchevsky N, Simonetti E. Relative population densities and predation of the common vampire bat (Desmodus rotundus) in natural and cattle-raising areas in north-east Argentina. Preventive Veterinary Medicine. 1992;14:13-20.
Moya MI, Pacheco LF, Aguirre LF. Relación de los ataques de Desmodus rotundus con el manejo del ganado caprino y algunas características del hábitat en la prepuna de Bolivia. Mastozoología Neotropical. 2015;22:73-84.
Gomes MN, Monteiro AM, Lewis N, Goncalves CA, Filho VS. Landscape risk factors for attacks of vampire bats on cattle in Sao Paulo, Brazil. Prev Vet Med. 2010;93(2-3):139-46. doi: 10.1016/j.prevetmed.2009.10.006.
License

Veterinaria México OA by Facultad de Medicina Veterinaria y Zootecnia - Universidad Nacional Autónoma de México is licensed under a Creative Commons Attribution 4.0 International Licence.
Based on a work at http://www.revistas.unam.mx
- All articles in Veterinaria México OA re published under the Creative Commons Attribution 4.0 Unported (CC-BY 4.0). With this license, authors retain copyright but allow any user to share, copy, distribute, transmit, adapt and make commercial use of the work, without needing to provide additional permission as long as appropriate attribution is made to the original author or source.
- By using this license, all Veterinaria México OAarticles meet or exceed all funder and institutional requirements for being considered Open Access.
- Authors cannot use copyrighted material within their article unless that material has also been made available under a similarly liberal license.



