Using spatial tools for high impact zoonotic agents’ surveillance design in backyard production systems from central Chile
Main Article Content
Abstract
Veterinaria México OA
ISSN: 2448-6760
Cite this as:
- Alegria Moran R, Lazo A, Urcelay S, Hamilton West C. Using spatial tools for high impact zoonotic agents’ surveillance design in backyard production systems from central Chile. Veterinaria México OA. 2017;4(1). doi: 10.21753/vmoa.4.1.435
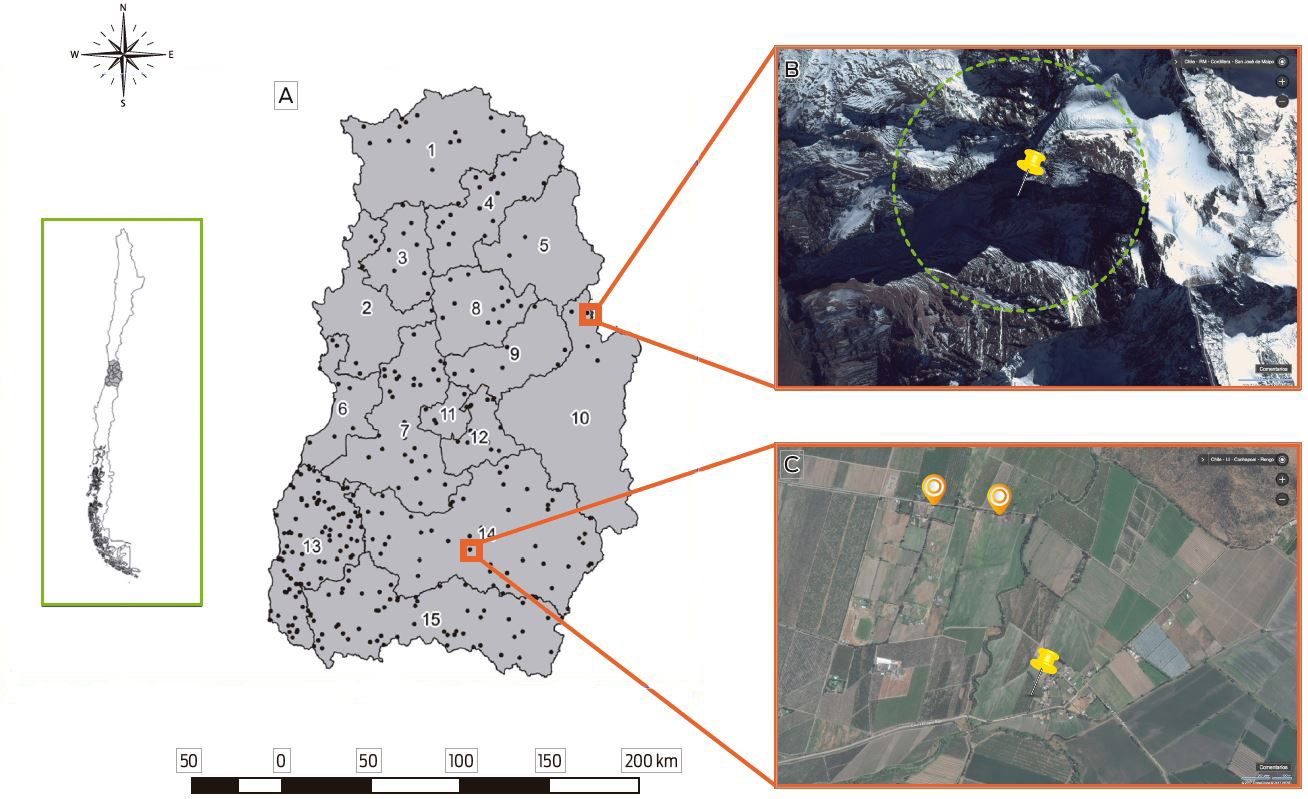
Specific locations of backyard production systems (BPSs) in Chile remain unclear, creating dificulties for designing surveillance activities for promptly detecting zoonotic agents with high impacts on health, such as avian influenza and Salmonella spp. This study aims to prove the use of spatial tools for improving the surveillance of BPSs in central Chile. A stratified and proportional random sampling was performed in 15 provinces of the Valparaiso, Libertador General Bernardo O’Higgins and Metropolitana regions. In this sampling, 329 BPSs were detected. In the first stage, 329 random sample points were allocated within the study area that searched for BPSs with poultry or swine breeding. Then, these random points were validated with remote sensing and in the field by searching for the presence of rural or semi-rural areas, nearby crops and houses or small towns within a 5 km radius around each point, while points allocated over hills or water sources (lakes or rivers) were discarded. Over 70 % of the sampling points were correctly allocated. In Los Andes, Cordillera and Chacabuco, less than 50 % of the points were allocated within feasible sampling areas.
From the total BPSs sampled, 89 % met the 5 km radius criteria, and in the provinces of Valparaiso, Cordillera and Cachapoal, over 20 % of the sampling points were outside the radius criteria. This study is the first in Chile to explore the locations and sanitary statuses of BPSs. Given the lack of knowledge about the specific locations of BPSs, their identification during field activities represents a high cost for the surveillance of pathogens. We argue that using spatial tools in BPS surveillance design is an important support for healthcare management.
Figure 1. Random sampling points by province assigned using ArcGIS® 10 and compatible zone detection by using free spatial tools. A. Study region with random sampling points. Study area and provinces: (1) Petorca; (2) Valparaiso, (3) Quillota; (4) San Felipe; (5) Los Andes; (6) San Antonio; (7) Melipilla; (8) Chacabuco; (9) Santiago; (10) Cordillera; (11) Talagante; (12) Maipo; (13) Cardenal Caro; (14) Cachapoal; (15) Colchagua. B. Random point (red pushpin) located in the Andes Mountains and 5 km searching area (yellow circle). C. Random point (red pushpin) and sampling candidate backyard farms (Yellow paddle) within less than 5 km.
Article Details
References
Conan A, Ponsich A, Luce Goutard F, Khiev R, Tarantola A, Sorn S, et al. A community-based education trial to improve backyard poultry biosecuri-ty in rural Cambodia. Acta Trop. 2013;125(3):294–302. doi: 10.1016/j. actatropica.2012.12.006.
Behravesh CB, Brinson D, Hopkins BA, Gomez TM. Backyard poultry flocks and salmonellosis: a recurring, yet preventable public health challenge. Clin Infect Dis. 2014;58(10):1432–8. doi: 10.1093/cid/ciu067.
Manning J, Gole V, Chousalkar K. Screening for salmonella in backyard chickens. Prev Vet Med. 2015;120(2):241–5. doi: 10.1016/j.prevetmed.2015.03.019.
McCune S, Arriola CS, Gilman RH, Romero MA, Ayvar V, Cama VA, et al. Interspe-cies interactions and potential influenza A virus risk in small swine farms in Peru. BMC Infect Dis. 2012;12(1):1–10. doi: 10.1186/1471-2334-12-58.
Jones BA, Grace D, Kock R, Alonso S, Rushton J, Said MY, et al. Zoonosis emer-gence linked to agricultural intensification and environmental change. Proc Natl Acad Sci USA. 2013;110(21):8399–404. doi: 10.1073/pnas.1208059110.
Short KR, Richard M, Verhagen JH, van Riel D, Schrauwen EJA, van den Brand JMA, et al. One health, multiple challenges: the inter-species transmission of in-fluenza A virus. One Health. 2015;1:1–13. doi: 10.1016/j.onehlt.2015.03.001.
Alexander DJ. A review of avian influenza in different bird species. Vet Microbiol. 2000;74(1–2):3–13. doi: 10.1016/S0378-1135(00)00160-7.
Zhu W, Shu Y. Genetic tuning of avian influenza A (H7N9) virus promotes vi-ral fitness within different species. Microbes Infect. 2015;17(2):118–22. doi: 10.1016/j.micinf.2014.11.010.
Nieto PA, Pardo-Roa C, Salazar-Echegarai FJ, Tobar HE, Coronado I, Riedel CA, et al. New insights about excisable pathogenicity islands in salmonella and their contribution to virulence. Microbes Infect. 2016;18(5):302–9. doi: 10.1016/j. micinf.2016.02.001.
Van Reeth K. Avian and swine influenza viruses: our current understanding of the zoonotic risk. Vet Res. 2007;38:243–60. doi: 10.1051/vetres:2006062.
Munster VJ, Baas C, Lexmond P, Waldenström J, Wallensten A, Fransson T, et al. Spatial, temporal, and species variation in prevalence of influenza A virus-es in wild migratory birds. PLoS Pathog. 2007;3(5):e61. doi: 10.1371/journal. ppat.0030061.
Retamal P, Fresno M, Dougnac C, Gutierrez S, Gornall V, Vidal R, et al. Ge-netic and phenotypic evidence of the Salmonella enterica serotype Enteritidis human-animal interface in Chile. Front Microbiol. 2015;6:464. doi: 10.3389/fmicb.2015.00464.
Bravo-Vasquez N, Di Pillo F, Lazo A, Jiménez-Bluhm P, Schultz-Cherry S, Ham-ilton-West C. Presence of influenza viruses in backyard poultry and swine in El Yali wetland, Chile. Prev Vet Med. 2016;134:211–5. doi: 10.1016/j. prevetmed.2016.10.004.
Pennycott TW, Park A, Mather HA. Isolation of different serovars of Salmonella enterica from wild birds in Great Britain between 1995 and 2003. Vet Rec. 2006;158(24):817–20. doi: 10.1136/vr.158.24.817.
Wiratsudakul A, Paul MC, Bicout DJ, Tiensin T, Triampo W, Chalvet-Monfray K. Modeling the dynamics of backyard chicken flows in traditional trade networks in Thailand: implications for surveillance and control of avian influenza. Trop Anim Health Prod. 2014;46(5):845–53. doi: 10.1007/s11250-014-0575-8.
Instituto Nacional de Estadísticas (INE). Censo Agropecuario. Santiago, Chile. 2007.
Dohoo R, Martin W, Stryhn H. Veterinary epidemiologic research. 2nd ed: Prince Edward Island, Canada: VER Inc.; 2010. 865 p.
Aronson J, Del Pozo A, Ovalle C, Avendaño J, Lavin A, Etienne M. Land use changes and conflicts in central Chile. In: Rundel PW, Montenegro G, Jaksic FM, editors. Landscape Disturbance and Biodiversity in Mediterranean-Type Ecosys-tems. Vol 136. Series Ecological Studies. Berlin, Heidelberg New York: Springer; 1998. p. 155–68.
Azócar G, Romero H, Sanhueza R, Vega C, Aguayo M, Muñoz MD. Urban-ization patterns and their impacts on social restructuring of urban space in chilean mid-cities: the case of Los Angeles, central Chile. Land Use Policy. 2007;24(1):199–211. doi: 10.1016/j.landusepol.2005.04.003.
Bamberger M, Rugh J, Church M, Fort L. Shoestring evaluation: designing impact evaluations under budget, time and data constraints. Am J Eval. 2004;25(1):5–37. doi: 10.1177/109821400402500102.
Salman M, editor. Animal disease surveillance and survey systems: methods and applications. Wiley-Blackwell; 2003. 222 p.
Ferguson JM, Langebrake JB, Cannataro VL, Garcia AJ, Hamman EA, Martche-va M, et al. Optimal sampling strategies for detecting zoonotic disease epi-demics. PLoS Comput Biol. 2014;10(6):e1003668. doi: 10.1371/journal. pcbi.1003668.
Saththasivam P, Voralu K, Ramli N, Mustapha MR, Omar J, Van Rostenberghe H. The effect of delayed transportation of blood samples on serum bilirubin values in neonates. Malays J Med Sci. 2010;17(3):27–31.
Hamilton-West C, Rojas H, Pinto J, Orozco J, Hervé-Claude LP, Urcelay S. Charac-terization of backyard poultry production systems and disease risk in the central zone of Chile. Res Vet Sci. 2012;93(1):121–4. doi: 10.1016/j.rvsc.2011.06.015.
Gonzales JL, Boender GJ, Elbers ARW, Stegeman JA, de Koeijer AA. Risk based surveillance for early detection of low pathogenic avian influenza out-breaks in layer chickens. Prev Vet Med. 2014;117(1):251–9. doi: 10.1016/j. prevetmed.2014.08.015.
Martin PAJ, Langstaff I, Iglesias RM, East IJ, Sergeant ESG, Garner MG. Assess-ing the efficacy of general surveillance for detection of incursions of livestock diseases in Australia. Prev Vet Med. 2015;121(3–4):215–30. doi: 10.1016/j. prevetmed.2015.06.017.
Rushton G. Public health, GIS, and spatial analytic tools. Annu Rev Public Health. 2003;24(1):43-56. doi: 10.1146/annurev.publhealth.24.012902.140843.
License

Veterinaria México OA by Facultad de Medicina Veterinaria y Zootecnia - Universidad Nacional Autónoma de México is licensed under a Creative Commons Attribution 4.0 International Licence.
Based on a work at http://www.revistas.unam.mx
- All articles in Veterinaria México OA re published under the Creative Commons Attribution 4.0 Unported (CC-BY 4.0). With this license, authors retain copyright but allow any user to share, copy, distribute, transmit, adapt and make commercial use of the work, without needing to provide additional permission as long as appropriate attribution is made to the original author or source.
- By using this license, all Veterinaria México OAarticles meet or exceed all funder and institutional requirements for being considered Open Access.
- Authors cannot use copyrighted material within their article unless that material has also been made available under a similarly liberal license.