Natural <em>Cysticercus fasciolaris</em> infection in rodents from a rural area in Yucatan, Mexico
Main Article Content
Abstract
Veterinaria México OA
ISSN: 2448-6760
Cite this as:
- Medina-Pinto RA, Torres-Castro MA, Medina-Pinto RA, Bolio-González ME, Rodríguez-Vivas RI. Natural Cysticercus fasciolaris infection in rodents from a rural area in Yucatan, Mexico. VetMéxOA;2019(2). doi: fmvz.24486760e.2019.2.590.
Cysticercus fasciolaris is the larval stage of Taenia taeniaeformis, a parasite that predominantly affects felines. It, however, has zoonotic significance since humans can be accidental hosts. Rodents and lagomorphs act as Intermediate hosts in this parasite’s life cycle. The aim of this study was to determine the natural occurrence of infection with Cysticercus fasciolaris in rodents from a rural area in Yucatan, Mexico. Rodents were captured in 40 dwellings and two neighboring areas of low deciduous forest. A total of 153 individuals of seven different species were captured: 65 Rattus rattus (42.5%), 44 Mus musculus (28.8%), 22 Heteromys gaumeri (14.4%), 11 Ototylomys phyllotis (7.2%), 9 Peromyscus yucatanicus (5.9%), 1 Peromyscus leucopus (0.6%), and 1 Sigmodon hispidus (0.6%). All animals were examined for evidence of parasitic liver infection. Rattus rattus was the only species to present positive Cysticercus fasciolaris infection (18.5%, 12/65). We thus concluded that there was no evidence of a transmission cycle with wild rodent species.
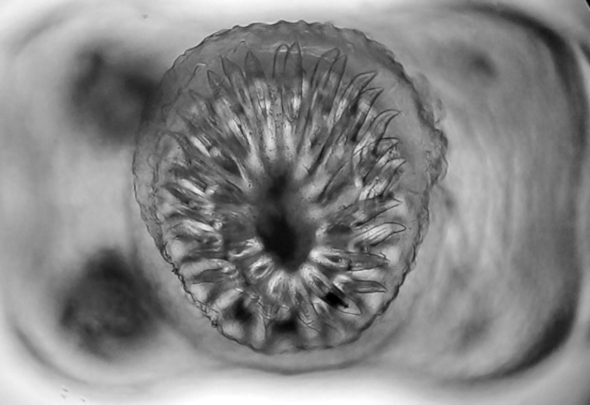
Figure 2. Scolex of Cysticercus fasciolaris with four lateral suckers and an armed rostel with two rows of hooks. Sample collected from a Rattus rattus captured in Cenotillo, Yucatán, Mexico.
Article Details
References
Krone O, Guminsky O, Meinig H, Herrmann M, Trinzen M, Wibbelt G. Endopar- asite spectrum of wild cats (Felis silvestris Schreber, 1777) and domestic cats (Felis catus L.) from the Eifel, Pfalz region and Saarland, Germany. Eur J Wild Res. 2008;54:95-100.
Stuti V, Swaid A, Deepesh S, Mir M. Parasitic ova and oocysts observed in in- testinal contents of a leopard (Panthera pardus)-A case report. J Vet Parasitol.2012;26(2):170-1.
Singla LD, Aulakh GS, Sharma R, Juyal PD, Singh J. Concurrent infection of Tae- nia taeniaeformis and Isospora felis in a stray kitten: a case report. Vet Med.2009: 54(2):81-3.
Custer JW, Pence DB. Ecological analyses of helminth populations of wild canids from the gulf coastal prairies of Texas and Louisiana. J Parasitol.1981;67(3):289-307.
Smith GC, Gangadharan B, Taylor Z, Laurenson MK, Brandshaw H, Hide G, Hughes JM, Dinkel A, Romig T, Craig PS. Prevalence of zoonotic import- ant parasites in the red fox (Vulpes vulpes) in Great Britain. Vet Parasitol.2003;118(1-2):133-42.
Hutchison WM. Studies on Hydatigera (Taenia) taeniaeformis. ll. Growth of the adult phase. Exp Parasitol. 1959;8:557-67.
Williams JF, Sheareer AM. Longevity and productivity of Taenia taeniaeformis in cats. Am J Vet Res. 1981;42(12):2182-3.
Karim AJ. Scanning electron microscopy and histological morphology of Cys- ticercus fasciolaris which induced fibrosarcomas in laboratory rats. Ann Micro.2010;10:44-8.
Hinz E. Vergleichende Untersuchungen an der experimentellen Zystizerkose von Ratte und Maus. Z Tropenmed Parasitol. 1962;13(182):183-94.
Yildiz K, Doğanay A. The efffect of albendazole and praziquantel on Stro- bilocercus fasciolaris in experimentally infected mice. Turk J Vet Anim Sci. 2001;25(2001):287-94.
Moudgil AD, Singla LD, Gupta K, Daundkar PS, Vemu B. Histopathological and morphological studies on natural Cysticercus fasciolaris infection in liver of Wis- tar rats. J Parasit Dis. 2016;40(2):255-8.
Miyazaki I. An illustrated book of Helminthic zoonoses. 1a ed. Tokio (Japan): International Medical Foundation of Japan; 1991.
Ekanayake S, Warnasuriya ND, Samarakoon PS, Abewickrama H, Kuruppuarach- chi ND, Dissanaike AS. An unusual ‘infection’ of a child in Sri Lanka, with Taenia taeniaeformis of the cat. Ann Trop Med Parasitol. 1999;93(8):869-73.
Oryan A, Alidadi S. Public health concerns of Taenidae and their metacestodes. Trop Med Surg. 2015:3(1):e123.
Pakdel N, Naem S, Rezaei F, Chalehchaleh AA. A survey on helminthic infection in mice (Mus musculus) and rats (Rattus norvegicus and Rattus rattus) in Ker- manshah, Iran. Vet Res Forum. 2013;4(2):105-9.
Sharma R, Tiwari K, Birmingham K, Armstrong E, Montanez A, Guy R, Sepúlveda Y, Mapp-Alexander V, DeAllie C. Cysticercus fasciolaris in Brown Rats (Rattus norvegicus) in Grenada, West Indies. J Parasitol Res. 2017;2017:1723406.
Bonfin TCB. Algumas observacoes sobre infeccao natural em Rattus norvegicus por Cysticercus fasciolaris (Eucestoda: Taeniidae). Rev Bras Parasitol Vet.2001;10(2):79-82.
Lee BW, Jeon BS, Kim HS, Kim HC, Yoon BI. Cysticercus fasciolaris infection in wild rats (Rattus norvegicus) in Korea and formation of cysts by remodeling of collagen fibers. J Vet Diagn Invest. 2016;28(3):263-70.
Wanas MUA, Shehera KK, Rashed AA. Larval occurrence of Hydatigera taeniae- formis Batsch (1786) (Cestoda: Taeniidae) in the liver of wild rodents in Egypt. J Egypt Soc Parasitol. 1993;23(2):381-8.
Malsawmtluangi C, Prasad KP, Biswal DK, Tandon V. Morphological and mo- lecular identification of the metacestode parasitizing the liver of rodent hosts in bamboo growing areas of mizoram, northeast India. Bioinformation.2011;7(8):393-9.
Sinniah B, Narasiman M, Habib S, Bei OG. Prevalence of Calodium hepaticum and Cysticercus fasciolaris in urban rats and their histopathological reaction in the livers. J Vet Med. 2014;2014:172829.
Ribas A, Saijuntha W, Agatsuma T, Thongjun C, Lamsan K, Poonlaphdech S. Helminths in rodents from wet markets in Thailand. Helminthologia.2016;53(4):326-30.
Pulido-Flores G, Moreno-Flores S, Monks S. Helminths of rodents (Rodentia: muridae) from Metztitlán, San Cristóbal, and Rancho Santa Elena, Hidalgo, Mex- ico. Comp Parasitol. 2005;72(2):186-92.
Rodríguez-Vivas RI, Panti-May JA, Parada-López J, Hernández-Betancourt SF, Ruiz-Piña HA. The occurrence of the larval cestode Cysticercus fasciolaris in rodent populations from the Cuxtal ecological reserve, Yucatan, Mexico. J Hel- minthol. 2011;85(4):458-61.
Panti-May JA, Hernández-Betancourt SF, Rodríguez-Vivas RR, Robles MR. Infec- tion levels of intestinal helminths in two commensal rodent species from rural households in Yucatan, Mexico. J Helmintol. 2013;89(1):42-8.
Secretaría de Desarrollo Social (SEDESOL). Unidad de microrregiones; Cédu- las de información municipal, Cenotillo, Yucatán. [Internet]. 2017 [citado 03 febrero 2018]; Disponible en: https://www.gob.mx/cms/uploads/attachment/ file/47145/Yucatan_012.pdf
Instituto Nacional para el Federalismo y el Desarrollo Municipal (INAFED). Cenotillo; Enciclopedia de los municipios y delegaciones de México. [Internet]. 2017 [citado 03 febrero 2018]. Disponible en: http://www.inafed.gob.mx/work/enciclopedia/EMM31yucatan/municipios/31012a.html
Sikes RS, Gannon WL, the American Care and Use Committee of the Amer- ican Society of Mammalogists. Guidelines of the American Society of Mam- malogists for the use of wild mammals in research and education. J Mammal. 2011;92(1):235-53.
Torres-Castro M, Gutiérrez-Ruiz E, Hernández-Betancourt S, Peláez-Sánchez R, Agudelo-Flórez P, Guillermo-Cordero L, Puerto FI. First molecular evidence of Leptospira spp. in synanthropic rodents captured in Yucatan, Mexico. Revue Méd Vét. 2014;165(7-8):213-8.
Torres-Castro M, Cruz-Camargo B, Medina-Pinto R, Reyes-Hernández B, Mo- guel-Lehmer C, Medina R, et al. Detección molecular de leptospiras patógenas en roedores sinantrópicos y silvestres capturados en Yucatán, México. Biomed- ica. 2018;38(Supl 2):51-8.
Reid FA. A field guide to the mammals of Central America and Southeast Mexi- co. 1a ed. New York (USA): Oxford University Press; 1997.
Leary S, Underwood W, Anthony R, Cartner S, Corey D, Grandin T, et al. AVMA guidelines for the euthanasia of animals. 2013 ed. Schaumburg, Illinois: Ameri- can Veterinary Medical Association; 2013.
Bowman DD, Lynn RC, Eberhard ML. Georgis’ parasitology for veterinarians. 10ª ed. Saint Louis, Missouri: Elsevier Saunders; 2014.
Widmer D, Jurczynski K. Infection with the strobilocercus of Taenia taeniaeform- is in a Malagasy giant jumping rat (Hypogeomys antimena). J Zoo Wild Med. 2012;43(4):914-21.
Theis J, Schwab R. Seasonal prevalence of Taenia taeniaeformis: relationship to age, sex, reproduction and abundance of an intermediate host (Peromyscus maniculatus). J Wild Dis. 1992;28(1):42-50.
Sosa-Escalante X, Hernández S, Segovia A, Sánchez-Cordero V. First record of the coyote, Canis latrans (Carnivora: Canidae) in the Yucatán Peninsula, Mexi- co. Southwest Nat. 1999;42(4):494-5.
Faller-Menéndez JC, Urquiza-Haas T, Chávez C, Johnson S, Ceballo G. Registros de mamíferos en la reserva privada el zapotal, en el noreste de la península de Yucatán. Rev Mex Mastozool. 2005;9:128-40.
Hernández-Pérez E, Reyna-Hurtado R, Castillo-Vela GC, Sanvicente-López M, Moreira-Ramírez FJ. Fototrampeo de mamíferos terrestres de talla mediana y grande asociados a petenes del noroeste de la península de Yucatán, México. Therya. 2007;6(3):559-74.
Chávez C, Zarza H. Distribución potencial del hábitat del jaguar y áreas de conflicto humano-jaguar en la península de Yucatán. Rev Mex Mastozool. 2009;13(1):46-62.
Panti-May JA, Hernández-Betancourt S, Ruiz-Piña H, Medina-Peralta S. Abun- dance and population parameters of commensal rodents present in rural house- holds in Yucatan, Mexico. Int Biodeterior Biodegradation. 2012;66(1):77-81.
Brandt JRA, Sewell MMH. Varying infectivity of Taenia taeniaeformis for rats and mice. Vet Res Commun. 1981;5(2):187-91.
Nonaka N, Iwaki T, Okamoto M, Ooi HK, Ohbayashi M, Kamiya M. Infectivi- ties of four isolates of Taenia taeniaeformis to various rodents. J Vet Med Sci. 1994;56(3):565-7.
Musoke AJ, Williams JF, Leid RW, Williams CSF. The immunological response of the rat to infection with Taenia taeniaeformis. IV. Immunoglobulins in- volved in passive transfer of resistance from mother to offspring. Immunology. 1975;29(5):845-53.
Singh BB, Rao BV. Experimental infection of Cysticercus fasciolaris in laboratory animals. Ann Parasitol Hum Comp. 1971;46(1):11-4.
Greenfield SH. Age resistance of the albino rat to Cysticercus fasciolaris. J Para- sitol. 1942;28(3):207-11.
License

Veterinaria México OA by Facultad de Medicina Veterinaria y Zootecnia - Universidad Nacional Autónoma de México is licensed under a Creative Commons Attribution 4.0 International Licence.
Based on a work at http://www.revistas.unam.mx
- All articles in Veterinaria México OA re published under the Creative Commons Attribution 4.0 Unported (CC-BY 4.0). With this license, authors retain copyright but allow any user to share, copy, distribute, transmit, adapt and make commercial use of the work, without needing to provide additional permission as long as appropriate attribution is made to the original author or source.
- By using this license, all Veterinaria México OAarticles meet or exceed all funder and institutional requirements for being considered Open Access.
- Authors cannot use copyrighted material within their article unless that material has also been made available under a similarly liberal license.



